Abstract
Inherited gain-of-function mutations in PIK3CD encoding PI3Kδ (phosphoinositide 3-kinase delta) lead to accumulation of senescent T cells, lymphadenopathy, splenomegaly and immune-deficiency (Activated PI3Kδ Syndrome, APDS). Clinical manifestations include multilineage cytopenias and susceptibility to B cell Non-Hodgkins Lymphoma.
We recently reported use of leniolisib (CDZ173), a novel, potent and selective oral PI3Kd inhibitor in six APDS patients in a 12-week, open-label, multi-center, within-subject dose-escalation study (Rao et al, Blood 23 November 2017). Leniolisib was safe and well tolerated and led to a dose-dependent reduction in PI3K/AKT pathway activity and improved the immune dysregulation with normalization of circulating transitional and naïve B cells and reduction in PD-1+CD4+ and senescent CD57+CD8+ T cells. Elevated serum IgM and other biomarkers including IFNg, TNF, CXCL13 and CXCL10 normalized or decreased with leniolisib. Cytopenias and lymphoproliferation improved in all patients with index lymph node sizes and spleen volumes reduced by 39% and 40%, respectively.
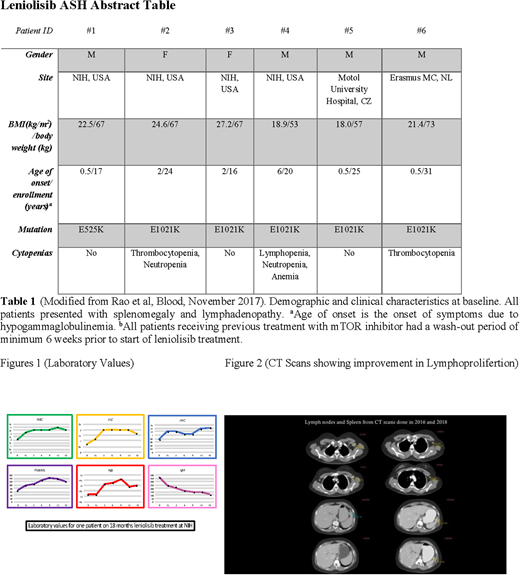
Here we report the long term follow up safety and efficacy data in all six patients (Table 1) who have continued treatment with leniolisib in an extension study at 70 mg b.i.d (ClinicalTrials.gov number NCT02859727). These patients have been treated for up to 949 days (as of July 20th 2018). Three of them were lymphoma survivors. Three patients were treated with rapamycin prior to starting leniolisib. No patient in the 12-week clinical trial or in the long term extension study has experienced any significant adverse events. Notably, side effects prevalent with mTOR or other PI3K inhibitors such as diarrhea/colitis, skin rashes, susceptibility to infections or liver enzyme elevation were absent. Three patients have stopped immunoglobulin supplementation as a reflection of the normalization of their B cell function. Cytopenias, including anemia, thrombocyopenia, neutropenia and lymphopenia have continued to improve. (Figure 1: Showing representative improvement of laboratory data including normalization of IgM for patient 002, following 18 months of treatment with leniolisib; Figure2: Showing improvement of lymphoproliferation in CT scans from 2016 and 2018 for Patient 003. This patient stopped leniolisib for 15 months from October 2016 until January 2018 leading to worsening lymphoproliferation that improved promptly on resumption of therapy). Available data on longterm safety, effects on lymphoproliferation and immune dysregulation in the ongoing leniolisib extension study will be shared during the meeting.
The results support safe and efficacious long term inhibition of PI3Kδ protein as a new targeted therapeutic approach in APDS and other disorders of nonmalignant lymphopoliferation associated with hyperactive PI3Kinase pathway.
Rao:novartis: Research Funding. Dalm:Novartis: Research Funding. Sediva:Novartis: Research Funding. van Hagen:Novartis: Research Funding. Cabanski:Novartis: Employment. Valentin:Novartis: Employment. de Buck:Novartis: Employment. Kalis:Novartis: Employment. Hasselberg:Novartis: Employment. Burkhart:Novartis: Employment. Kucher:Novartis: Employment. Sloth:Novartis: Employment. Uzel:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal